FAU Engineers and Sensing Institute Map the Brain's Blood Flow
The study offers new insights into brain protection and potential breakthroughs in diagnosing stroke, Alzheimer’s and traumatic brain injuries.
Healthy brain function relies on a steady supply of blood. Disruptions in blood flow are linked to major neurological conditions like stroke, Alzheimer’s disease (AD), and traumatic brain injuries. But understanding how the brain fine-tunes this flow – especially across its smallest blood vessels – remains a challenge.
The brain’s blood supply includes a vast network of vessels, ranging from large arteries to microscopic capillaries. Between these lie transitional zone (TZ) vessels – such as penetrating arterioles, precapillary arterioles, and capillary sphincters – that bridge the gap and may play a big role in regulating flow. But their exact contribution, particularly during increased brain activity, remains a subject of scientific debate.
To explore these dynamics, researchers from the College of Engineering and Computer Science at Florida Atlantic University and the FAU Sensing Institute (I-SENSE) developed a highly detailed computer model of the mouse brain’s vasculature, treating each vessel segment as a tiny, adjustable valve.
The model simulates how brain blood vessels respond to two key factors: hemodynamics, the movement of blood through the vessels, and vasodynamics, the way vessels actively change shape in response to that flow. By integrating both processes, the model reveals how different components of the brain’s vascular system work together to maintain stable blood flow – even as conditions shift, such as blood pressure fluctuation or increased activity in specific brain regions. To evaluate its accuracy, the researchers compared the model’s predictions against real biological data.
Results of the study, published in PLOS ONE, show that brain blood vessels operate in four distinct phases based on blood pressure. At very low pressures, blood flow drops below optimal levels. As pressure rises, the system enters a “sweet spot” where flow remains steady across a wide range. But beyond a certain threshold, vessels lose control and flow increases rapidly – potentially stressing or damaging delicate vessel walls.
“Not all vessels play equal roles in maintaining healthy circulation in the brain,” said Ramin Pashaie, Ph.D., senior author, professor, FAU Department of Electrical Engineering and Computer Science and Department of Biomedical Engineering, and an I-SENSE faculty fellow. “Our model shows that transitional vessels – those between arteries and capillaries – make the most critical adjustments to protect the brain and ensure a consistent supply of oxygen and nutrients. It also helps explain how the brain stays protected across different physiological conditions. The vessel walls themselves – particularly the endothelial cells – can only constrict so much. Once that limit is reached, the system loses some control over blood flow, which can lead to increased stress on vessel walls and may contribute to disease or injury.”
The model also captured how blood flow increases during brain activity – known as functional hyperemia – with different types of vessels taking the lead depending on their location. In outer layers, sphincters and TZ vessels perform most of the regulatory work; deeper in the brain, penetrating arterioles take over.
By accurately modeling how the brain manages blood pressure and oxygen delivery across its microvascular system, the team’s work lays a foundation for developing better diagnostic tools, smarter simulations, and more effective treatments for a wide range of brain conditions.
“As engineers, we’re using computation to reveal what biology alone can’t always show,” said Pashaie, who is also a member of the FAU Stiles-Nicholson Brain Institute. “Our model shows that healthy brains are equipped with fine-tuned systems for protecting themselves – but when those systems fail, even small changes in pressure or vessel function can have big consequences.”
The study underscores the power of cross-disciplinary collaboration between engineering, neuroscience and computational modeling. The team now hopes to refine the model further and eventually apply it to human brain data.
This model is the most recent research phase in this FAU engineering team’s pursuit of developing a procedure for early detection of AD through a simple eye exam. Based on experimental observations, the team has hypothesized that changes in the blood flow regulatory system of the brain occur at very early stages of AD, accompanied by ocular impairments. Similar changes in the blood flow regulatory system of retina are expected, which is accessible for imaging even in humans. By understanding how the blood flow regulatory system changes under AD and how these changes are correlated with the changes in retina, retinal vasculature can be imaged non-invasively. The imaging data can be processed by artificial intelligence algorithms to diagnose AD and classify the stage and progression of the disease.
“This latest research provides novel and vital insights into the complex mechanisms that regulate blood flow in the brain – particularly how tiny vessels adapt to changing conditions to keep the brain nourished and protected,” said Stella Batalama, Ph.D., dean of the FAU College of Engineering and Computer Science. “These findings don’t just advance our understanding of basic physiology; they have real potential to transform how we approach neurological disorders as well as brain trauma. By combining advanced computer modeling with biological insight, our researchers are pushing the boundaries of what’s possible in brain health.”
Study co-authors are Hadi Esfandi, first author and a graduate research assistant, FAU I-SENSE; Mahshad Javidan, Ph.D., a data scientist and FAU Ph.D. graduate from the College of Engineering and Computer Science; and Rozalyn M. Anderson, Ph.D., professor of geriatrics and gerontology, Department of Medicine, University of Wisconsin School of Medicine and Public Health.
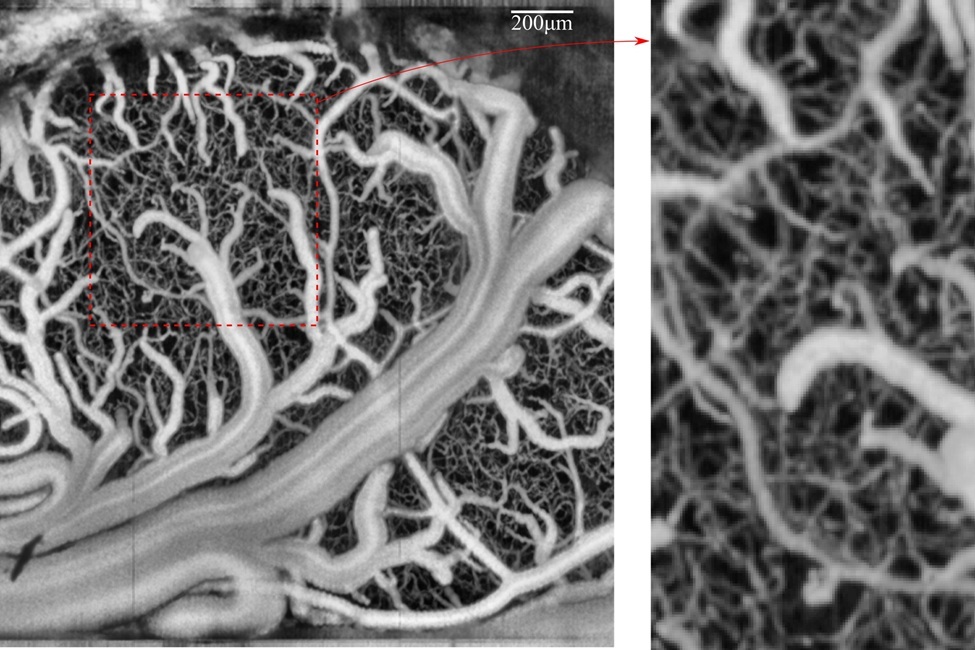
Network of vessels in the brain imaged by the researchers.
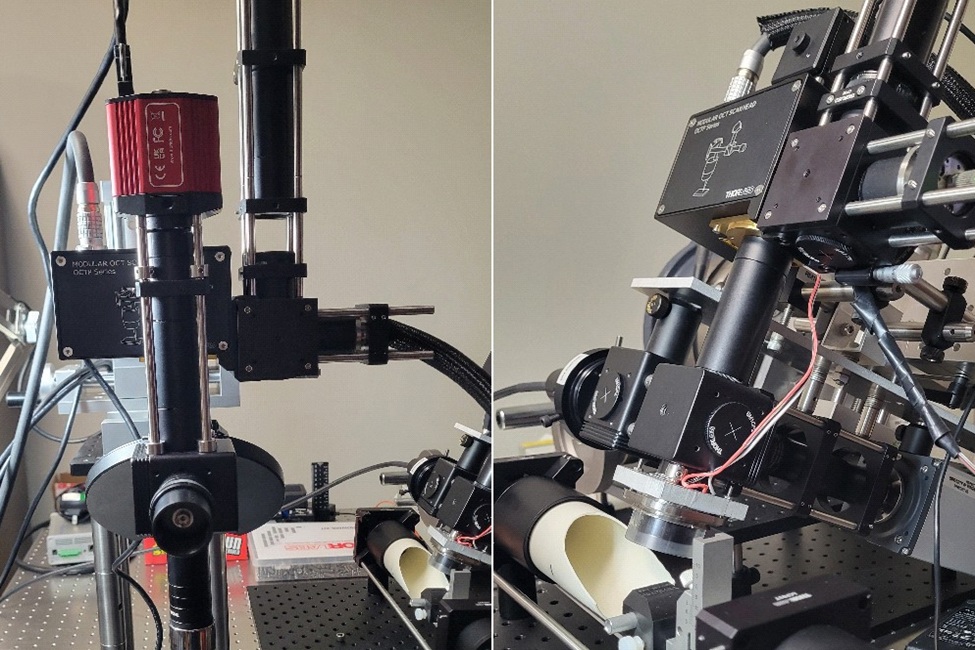
The imaging systems developed and used by the researchers.
-FAU-